Vitamin D, D-binding protein, free vitamin D and COVID-19 mortality in hospitalized patients
8th treatment shown to reduce risk in
October 2020 *, now known with p < 0.00000000001 from 120 studies, recognized in 8 countries.
|
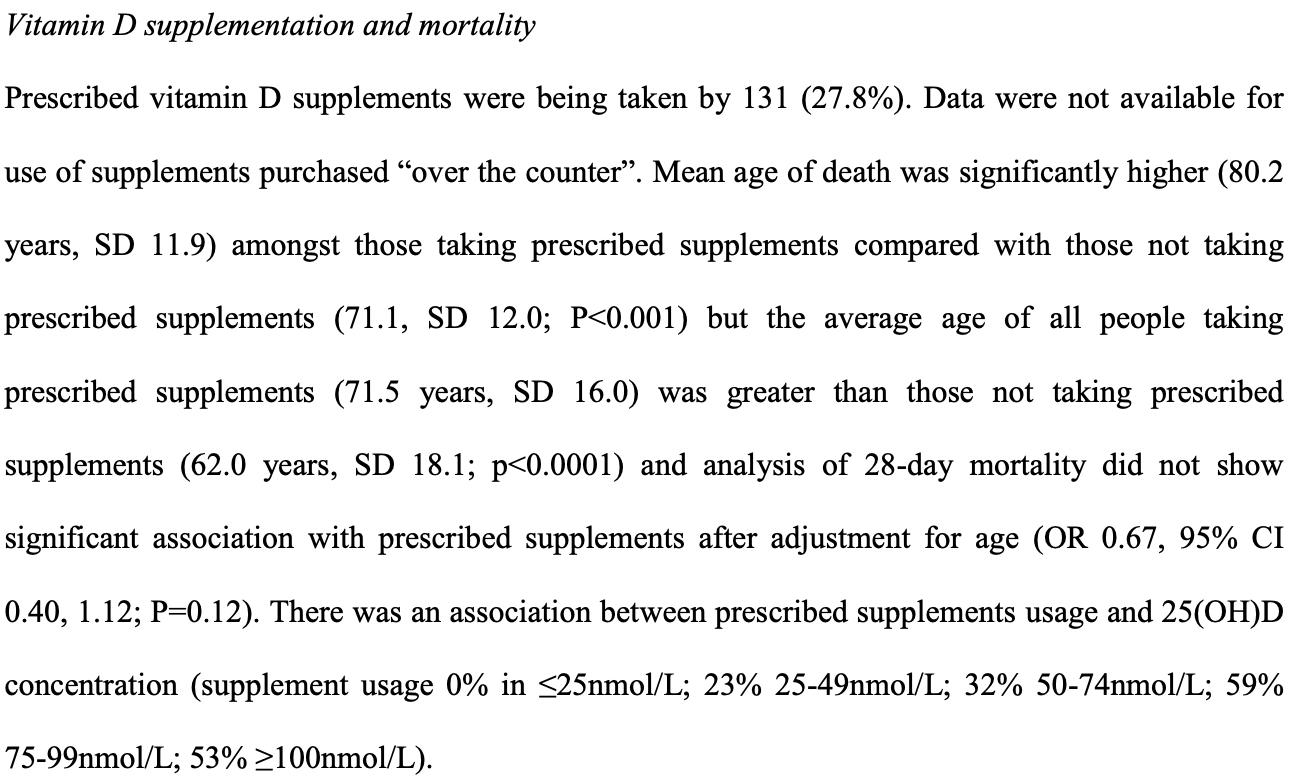
Retrospective 427 hospitalized COVID-19 patients in the United Kingdom, showing lower mortality with vitamin D supplementation (p=0.12), and higher mortality with both low and high vitamin D levels compared to a reference range of 50-74 nmol/L.
29 studies are RCTs, which show efficacy with
p=0.0000024.
|
risk of death, 27.3% lower, RR 0.73, p = 0.12, treatment 31 of 131 (23.7%), control 80 of 336 (23.8%), adjusted per study, odds ratio converted to relative risk, prescribed supplement use, multivariable.
|
|
risk of death, 49.7% lower, RR 0.50, p = 0.02, high D levels 16 of 115 (13.9%), low D levels 33 of 118 (28.0%), NNT 7.1, adjusted per study, inverted to make RR<1 favor high D levels, odds ratio converted to relative risk, 50-74 nmol/L vs. <25nmol/L, multivariable, outcome based on serum levels.
|
|
risk of death, 39.7% lower, RR 0.60, p = 0.07, high D levels 16 of 115 (13.9%), low D levels 38 of 157 (24.2%), NNT 9.7, adjusted per study, inverted to make RR<1 favor high D levels, odds ratio converted to relative risk, 50-74 nmol/L vs. 25-49nmol/L, multivariable, outcome based on serum levels.
|
|
Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates
|
Subramanian et al., 31 Jan 2022, prospective, United Kingdom, peer-reviewed, 16 authors, dosage not specified.
Abstract: Vitamin D, D-binding protein, free vitamin D and COVID-19
mortality in hospitalized patients.
Short title: Vitamin D and COVID-19 mortality
PT
Martin Hewison5, Rene F Chun6, Andrea Jorgensen7, Paul Richardson1, Darshan Nitchingham1,
RI
Joseph Aslan1, Maya Shah1, Coonoor R Chandrasekar8, Amanda Wood9, Mike Beadsworth10,
Department of Gastroenterology, Liverpool University Hospital Foundation NHS Trust, Liverpool,
N
U
1
SC
Munir Pirmohamed11
Department of Cellular and Molecular Physiology, Institute of Translational Medicine, University
M
2
A
UK
3
IT
ED
of Liverpool
Department of Clinical Chemistry, Liverpool University Hospital Foundation NHS Trust,
Liverpool, UK
Department of Biostatistics, Institute of Translational Medicine, University of Liverpool,
ED
4
N
Liverpool, UK
Institute of Metabolism and Systems Research, University of Birmingham
6
Department of Orthopaedic Surgery, University of California, Los Angeles
7
Department of Health Data Science, University of Liverpool, Liverpool, UK
8
Department of Orthopaedic Surgery, Liverpool University Hospital Foundation NHS Trust,
IN
A
L
U
5
Department of Clinical Pharmacology, Liverpool University Hospital Foundation NHS Trust,
O
RI
9
G
Liverpool, UK
Liverpool, UK
© Crown copyright 2022. This article contains public sector information licensed under the Open Government Licence v3.0
(http://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/).
Sreedhar Subramanian1, 2 ¶, Jonathan M Rhodes2, , Joseph M Taylor3, Anna M Milan3, Steven Lane4,
10
Tropical and Infectious Diseases Unit, Liverpool University Hospital Foundation NHS Trust,
Liverpool, UK
11
Department of Molecular and Clinical Pharmacology, University of Liverpool
¶
Corresponding author:
Consultant Gastroenterologist and Honorary Senior Lecturer
PT
Department of Gastroenterology
RI
Liverpool University Hospital Foundation NHS Trust and University of Liverpool
SC
Prescot Street
Liverpool L7 8XP, UK
N
U
Email: sreedhar.subramanian@liverpoolft.nhs.uk
A
Acknowledgements: None
M
Registration: The study protocol was approved by the London-Surrey Research Ethics Committee
IT
ED
(20/HRA/2282).
Funding Source: This research was funded by an internal departmental grant from Liverpool
ED
University Hospitals NHS Foundation Trust. The funders had no input into study design or final
Guarantor of the article
A
L
U
Dr Sreedhar Subramanian
N
analysis.
IN
Author contributions: SS, JR, MP, JT and AM were involved in study design and initial drafting
G
of the manuscript. SL was involved in data analysis. All authors revised and approved the final
O
RI
version of the manuscript.
Sreedhar Subramanian, MD, FRCP
Conflict of interest: JMR with the University of Liverpool and Provexis UK, holds a patent for
use of a soluble fibre preparation as maintenance therapy for Crohn’s disease plus a patent for its
use in antibiotic-associated diarrhoea. Patent also held with the University of Liverpool and others
in relation to use of modified heparins in cancer therapy. SS has received speaker fees from MSD,
Actavis, Abbvie, Dr Falk pharmaceuticals, Janssen, Takeda, Boehringer-Ingelheim, Shire and
Abbvie, Dr Falk..
Please send us corrections, updates, or comments.
c19early involves the extraction of 100,000+ datapoints from
thousands of papers.
Community updates
help ensure high accuracy.
Treatments and other interventions are complementary.
All practical, effective, and safe
means should be used based on risk/benefit analysis.
No treatment or intervention is 100% available and effective for all current
and future variants.
We do not provide medical advice. Before taking any medication,
consult a qualified physician who can provide personalized advice and details
of risks and benefits based on your medical history and situation.
FLCCC and
WCH
provide treatment protocols.
Submit